Learning Outcomes
i. Students will be able to explain the concept of phenol synthesis and its importance.
ii. Students will understand the preparation of phenol from benzene sulfonic acid, chlorobenzene, acidic oxidation of cumene, and hydrolysis of diazonium salts.
iii. Students will be able to compare the advantages and disadvantages of different phenol preparation methods.
Introduction
Phenol, a versatile and industrially important organic compound, is widely used in the production of plastics, synthetic resins, and pharmaceuticals. Its synthesis from various precursors plays a crucial role in meeting the demand for this valuable chemical.
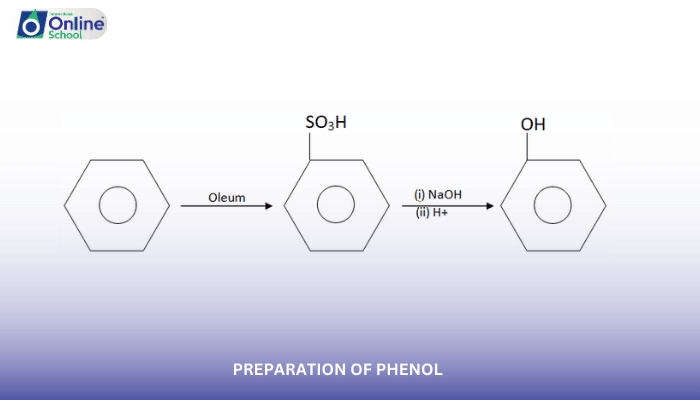
i. Preparation of Phenol from Benzene Sulfonic Acid
The preparation of phenol from benzene sulfonic acid involves a three-step process:
Sulfonation of Benzene: Benzene is treated with concentrated sulfuric acid to produce benzene sulfonic acid.
Conversion to Sodium Phenoxide: Benzene sulfonic acid is fused with sodium hydroxide to generate sodium phenoxide, the sodium salt of phenol.
Acidification of Sodium Phenoxide: Sodium phenoxide is acidified with sulfuric acid to obtain phenol.
Advantages of the Benzene Sulfonic Acid Method
- Relatively inexpensive starting materials
- Produces high yields of phenol
- Well-established and widely used method
Disadvantages of the Benzene Sulfonic Acid Method
- Requires high temperatures and pressures
- Generates large amounts of waste sulfuric acid
ii. Preparation of Phenol from Chlorobenzene
The Dow process is a common method for the synthesis of phenol from chlorobenzene. This method involves the reaction of chlorobenzene with sodium hydroxide under high pressure (320 atm) and temperature (623 K) to produce phenol and sodium chloride.
Advantages of the Dow Process
- High yields of phenol
- Relatively mild reaction conditions compared to the benzene sulfonic acid method
- Produces valuable byproducts (sodium chloride)
Disadvantages of the Dow Process
- Requires high pressures
- Generates large amounts of sodium chloride waste